The first medical device for preventing migraines

The U.S. Food and Drug Administration(FDA) allowed the marketing of Cefaly, an anti-migraine device. The device can be used as a preventive treatment for migraine and headaches.
Migraine can cause intense throbbing or a pulsing sensation in one area of the head. Migraines usually comes with nausea, vomiting, and extreme sensitivity to light and sound. Attacks can cause pain from hours to days. It can also be so severe that makes the body hard to function.
According to studies, nearly 12 percent of the U.S. population suffers from migraines. Another discovery also pointed out that women are three times more likely than men to suffer from it. Research on migraine has showed that the condition could lead to brain damage.
It is a great relief that finally a migraine preventing device is out in the market. Director of the Office of Device Evaluation (ODE) at the FDA's Center for Devices and Radiological Health (CDRH) Christy Foreman said, "This [device] may help patients who cannot tolerate current migraine medications for preventing migraines or treating attacks."
The Cefaly device looks like a small headband. It applies an electric current to the skin that stimulates branches of trigeminal nerve. This nerve is responsible for migraine headaches. It has a prevention program designed to increases the production of endorphins that blocks pain and provides relief during attacks.
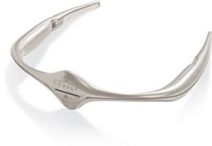
This device can also be used by children over 8 years old. Children below 8 years of age may use Cefaly if supervised by an adult. It is also is ideal for pregnant women. It is safer for nursing mothers to use, than to take dangerous pain killing medication.